
SF4 Lewis Structure How to Draw the Lewis Structure for SF4 YouTube
The SF4 Lewis structure refers to the arrangement of atoms and electrons in a molecule of sulfur tetrafluoride. In this structure, there is one sulfur atom bonded to four fluorine atoms. The Lewis structure helps us understand the bonding and electron distribution in a molecule. It shows the connectivity of atoms and the placement of lone pairs.

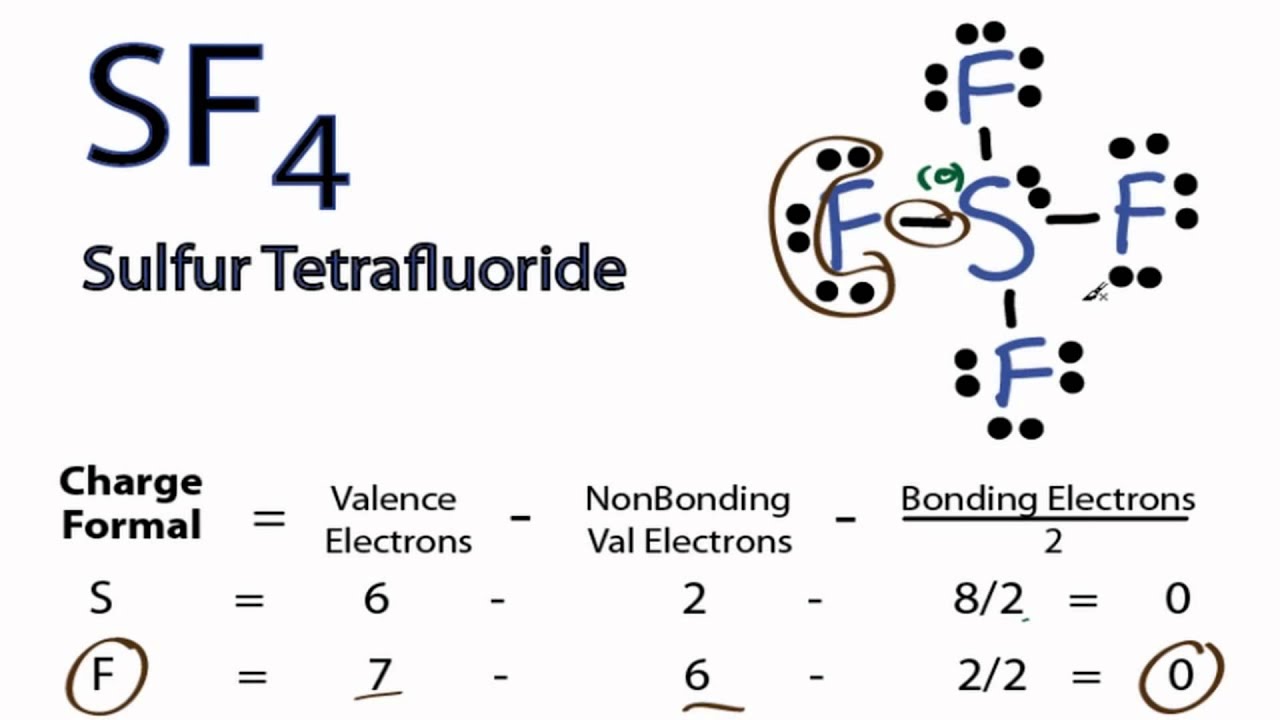
SF4 Lewis Structure ,Valence Electrons,Formal Charge,Octet Rule
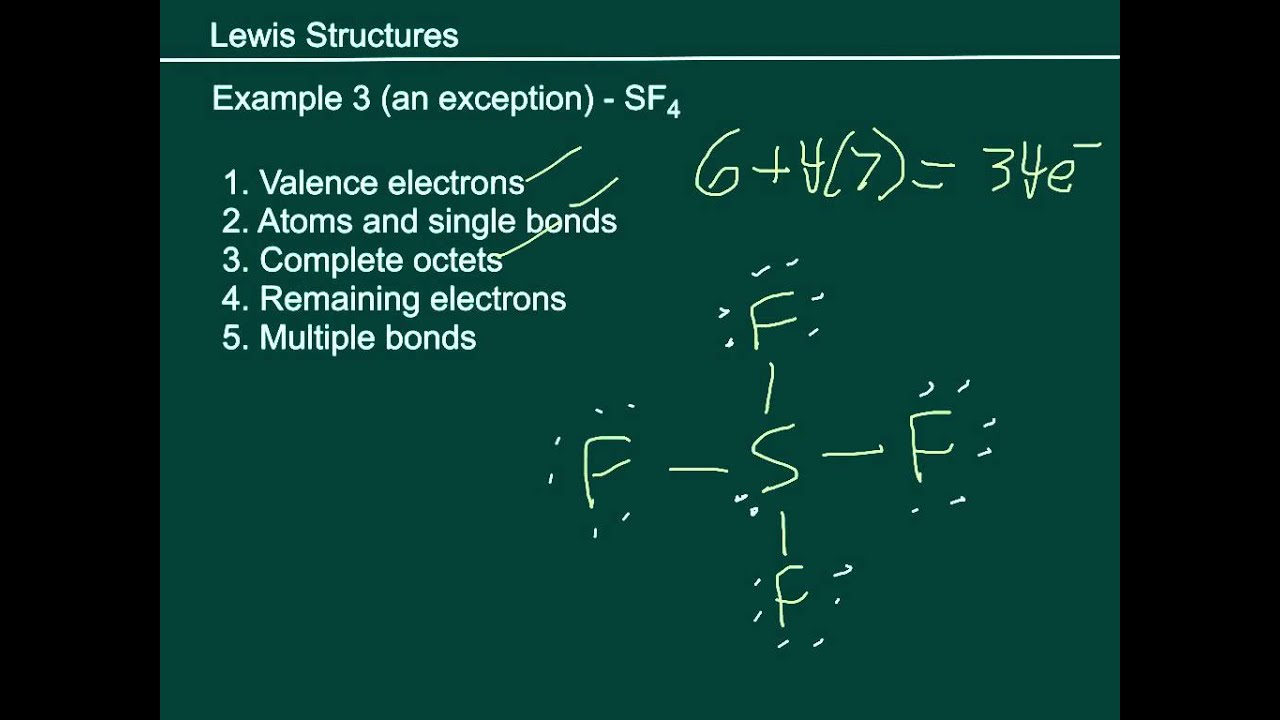
Drawing the Lewis Structure for SF 4. Viewing Notes: SF 4 is Lewis structure with Sulfur (S). Remember that Sulfur can hold more than 8 valence electrons.. Let's do the SF4 Lewis structure. On the periodic table, 6 valence electrons for Sulfur, 7 for Fluorine but we have 4 Fluorines; for a total of 34 valence electrons. We'll put the S, the.

Sf4 Polar or Nonpolar
A step-by-step explanation of how to draw the SF4 Lewis Structure (Sulfur Tetrafluoride).For the SF4 Lewis structure use the periodic table to find the total.

SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Techiescientist
SF4 Lewis Structure A. Definition and concept. The SF4 Lewis structure is a diagram that represents the arrangement of atoms and electrons in the molecule. The concept of valence electrons, which participate in chemical bonding as the outermost electrons, serves as the basis for it. The structure shows the central sulfur atom bonded to four.

How to draw SF4 Lewis Structure? Science Education and Tutorials
The SF4 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the SF4 molecule. The geometry of the SF4 molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory (VSEPR Theory), which states that molecules will choose the SF4 geometrical shape in which the electrons.

How to draw SF4 Lewis Structure? Science Education and Tutorials
Hello Guys!Today we are going to look at the Lewis Structure of SF4 ( Xenon Tetrafluoride )The Sulphur atom has six valence electrons in its outer shell and.
[Solved] Draw the Lewis structure of SF 4 showing all lone pairs. Identify... Course Hero
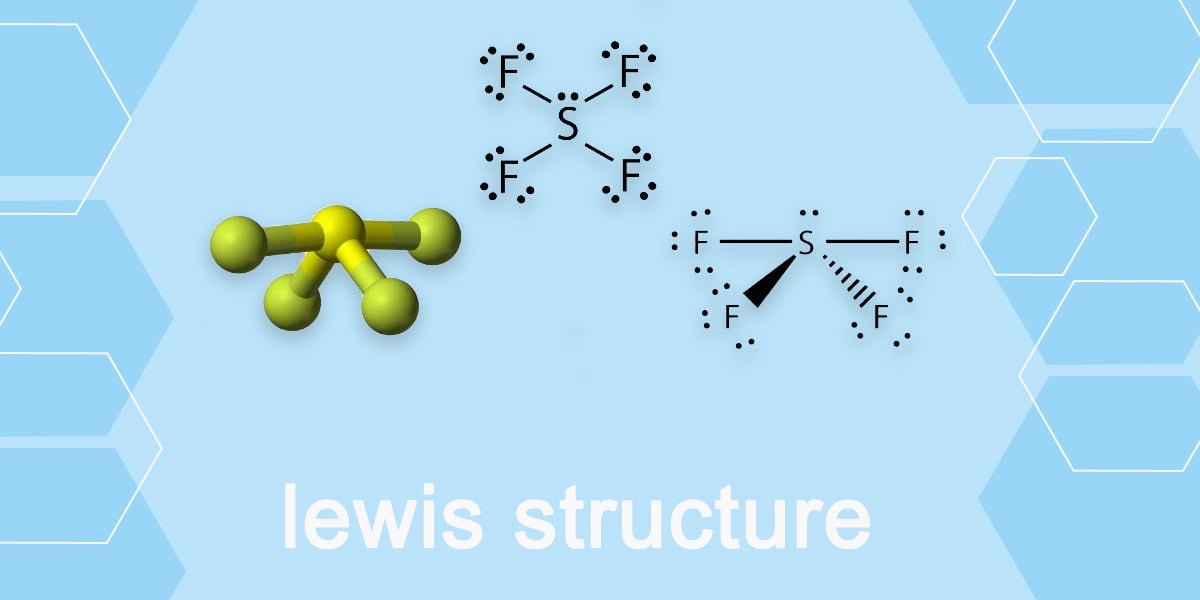
Lewis structure is used to show the bond formation in sulfur tetrafluoride. Sulfur is the least electronegative of the two. So, it will lie at the center of the molecule. Dash lines represent the four S-F single covalent bonds. Dots represent the lone pairs. VSEPR theory is used to predict the shape of the SF 4 molecule. According to this.
Lewis Structure Of Sf4 slidesharetrick
To draw the Lewis structure of SF4, we follow a step-by-step process. First, we arrange the atoms, then distribute the valence electrons, and finally, place them around the atoms to form bonds and lone pairs. 1. Determine the Total Number of Valence Electrons. To begin, count the total number of valence electrons in the SF4 molecule.

Is SF4 Polar or Nonpolar? Techiescientist
I quickly take you through how to draw the Lewis Structure of SF4, Sulfur TetraFluoride. I also go over formal charge, hybridization, shape and bond angle.**.

SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Techiescientist
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: The Lewis structure for SF4 is shown. What is the electron-pair geometry and the molecular geometry around the central atom? The Lewis structure for SF4 is shown. What is the electron-pair geometry and the molecular geometry around the.
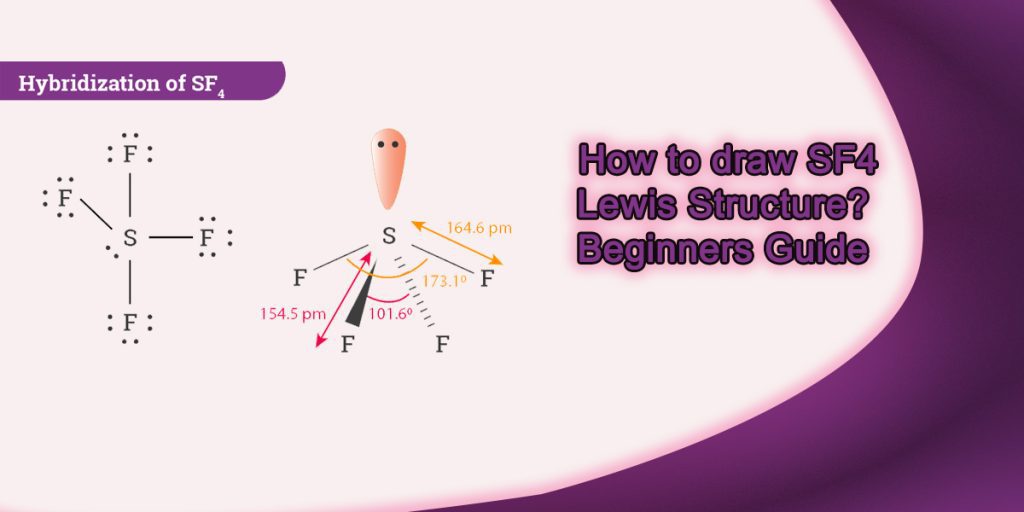
How to draw Sf4 Lewis Structure? Beginners Guide
Sulfur tetrafluoride (SF4) Lewis dot structure, molecular geometry or shape, electron geometry, bond angle, formal charge. SF 4 is the chemical formula for sulfur tetrafluoride, a colorless gas with a distinct rotten-egg-like odor. Its molar mass is 108.4 g/mol thus it is heavier than air. Sulfur tetrafluoride is a highly toxic gas that can.
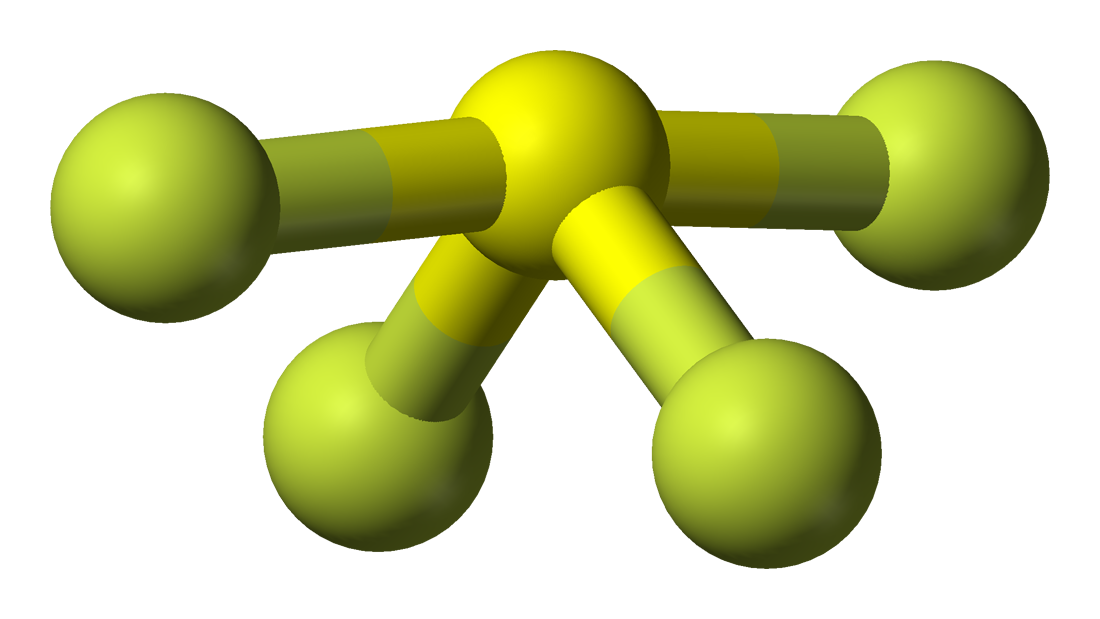
SF4 Molecular Geometry, Lewis Structure, Bond Angles and Polarity
SF4 Lewis Structure. Lewis structure is a pictorial representation of the bonds and valence electrons in the molecule. The bonds formed between two atoms are depicted using lines, whereas the valence electrons not forming any bonds are shown by dots. The valence electrons that participate in forming bonds are called bonding pairs of electrons.
Basic Lewis Structures SF4 YouTube
The Lewis structure helps one to understand the sharing of electrons between the central and neighboring atoms within a compound. Here the central atom is Sulfur and the neighboring atoms are Fluorine. This is the general idea of how and why Lewis structures are made. SF4 Lewis Structure. Let us look at how SF4's Lewis structure can be formed.
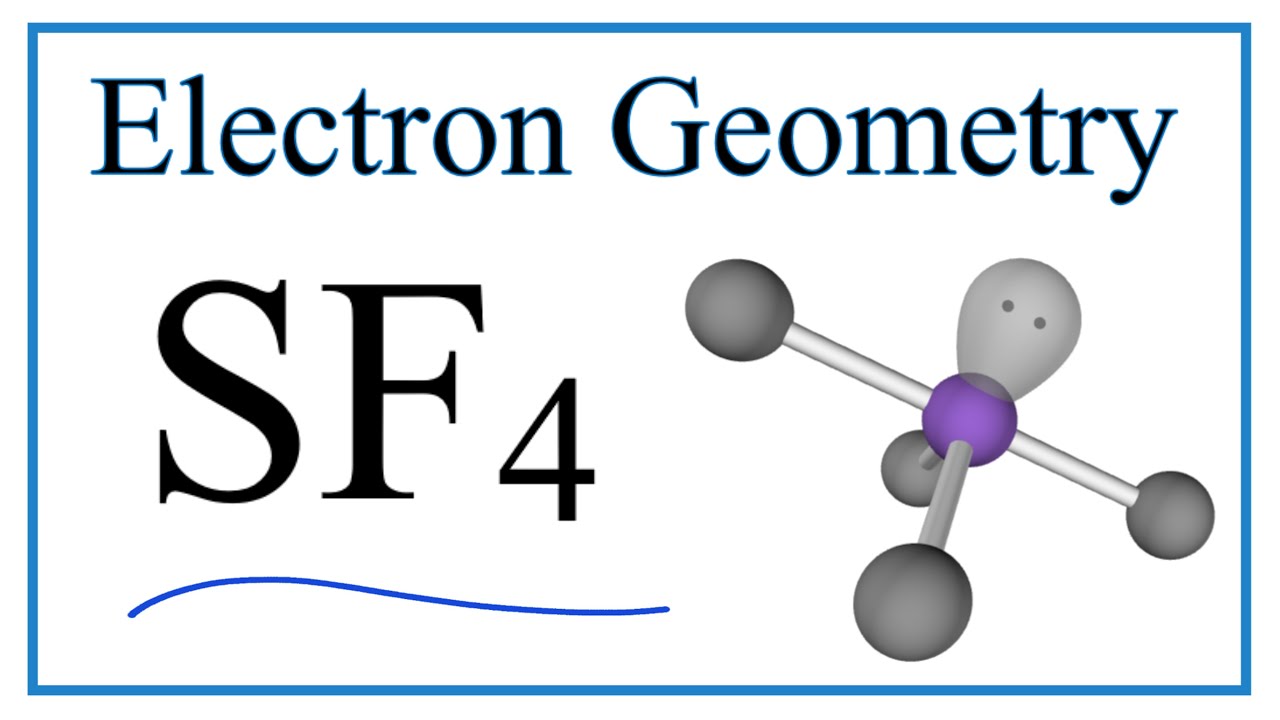
SF4 Electron Geometry (Sulfur tetrafluoride) YouTube
In SF4 lewis structure, each fluorine atom has joint with center sulfur atom. Also, there is a lone pair on sulfur atom. Sulfur has a valence of 6. Therefore sulfur becomes the center atom. In this tutorial, we will learn how to draw the lewis structure of SF4 step by step.

SF4 Lewis Structure How to Draw the Lewis Structure for SF4 YouTube
A step-by-step explanation of how to draw the SF4 Lewis Dot Structure (Sulfur tetrafluoride).For the SF4 structure use the periodic table to find the total n.
How to draw Sf4 Lewis Structure? Beginners Guide
The Lewis structure of SF4 indicates five regions of electron density around the sulfur atom: one lone pair and four bonding pairs: We expect these five regions to adopt a trigonal bipyramidal electron-pair geometry. To minimize lone pair repulsions, the lone pair occupies one of the equatorial positions..